NOVASYNC HYBRID SYSTEMTM (NOVASYNC), Grand Pharma’s global innovative intravascular dual-mode imaging system for coronary artery imaging, has been granted registration certificate for medical device by the National Medical Products Administration of the People’s Republic of China (NMPA) recently.
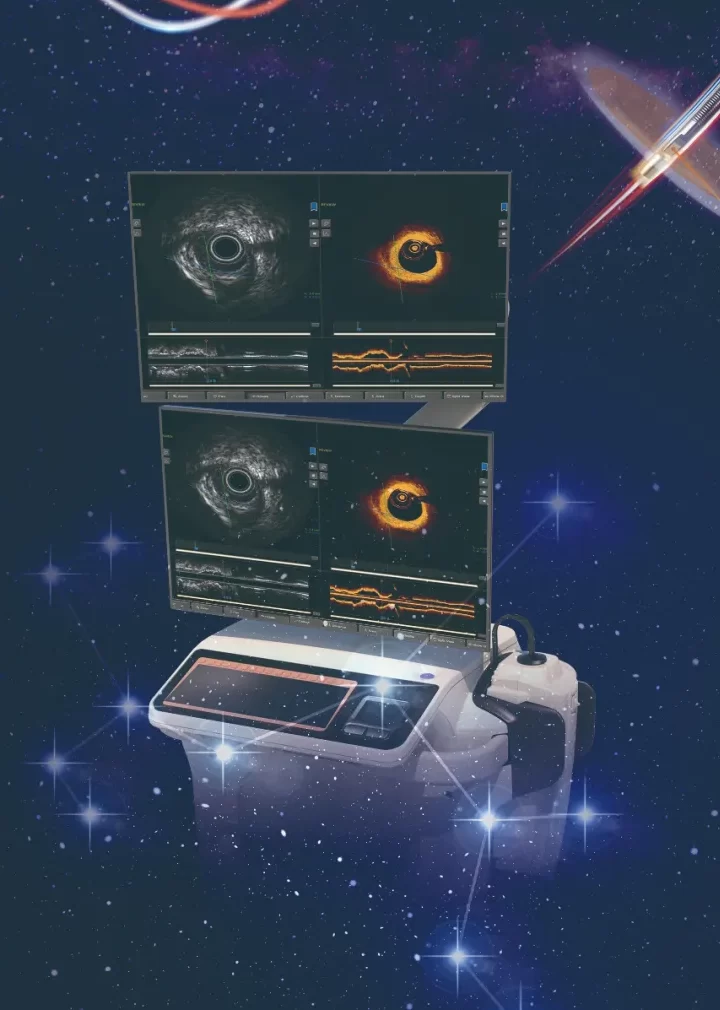
NOVASYNC is a domestic iteration of the intravascular dual-mode imaging device NOVASIGHT HYBRID SYSTEMTM (NOVASIGHT) imported by Grand Pharma. The approval of this product marks that our Group has successfully completed the technology transfer and full localization of NOVASIGHT. This is another milestone progress of our Group in the field of cerebrocardiovascular precision interventional diagnosis and treatment. NOVASYNC combines IVUS and OCT, thereby can simultaneously show the ultrasound and optical image with the same direction, axis and phase.
Optical coherence tomography (OCT) and intravascular ultrasound (IVUS) are the two most important intravascular imaging techniques in percutaneous coronary intervention (PCI) surgery, which can significantly improve the therapeutic effect of patients undergoing PCI surgery.
NOVASYNC inherits the excellent performance and high quality of NOVASIGHT, achieves compatibility between domestic and imported products, can further reduce the production cost of the product, and is expected to benefit more patients with coronary heart disease. The successful commercialization of NOVASYNC means that the Group has the ability to combine advanced foreign technologies with domestic superior production capacity in the field of medical devices. It also lays a solid foundation for future product upgrades of the Group in the field of cerebro-cardiovascular precision interventional diagnosis and treatment.





