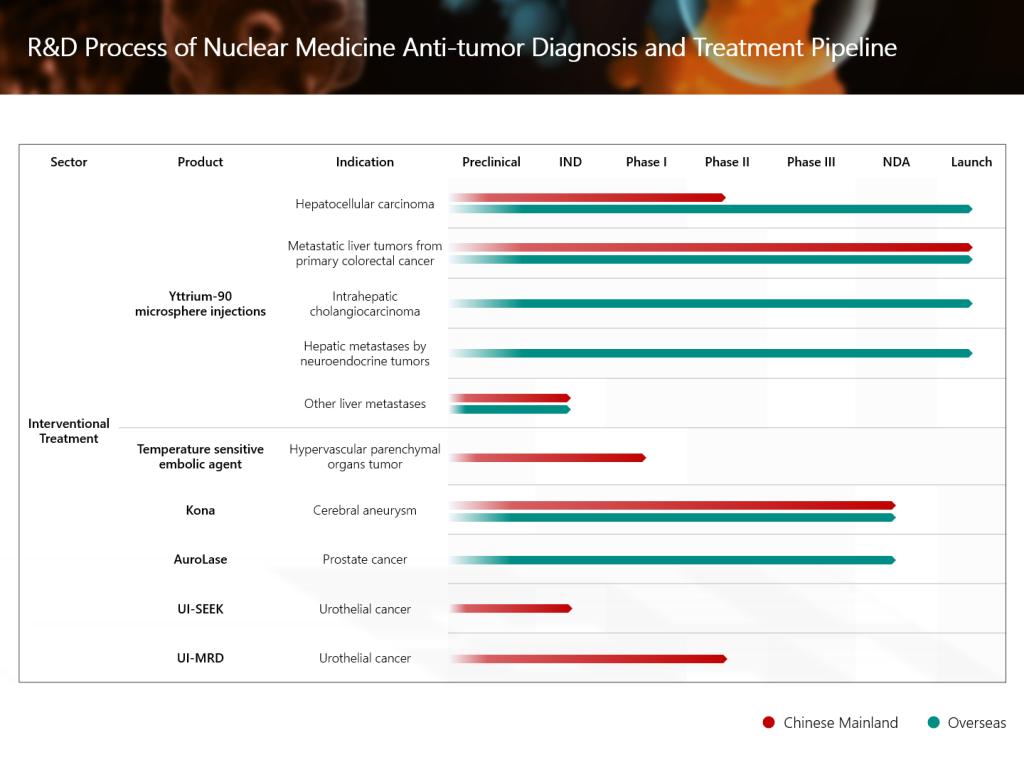
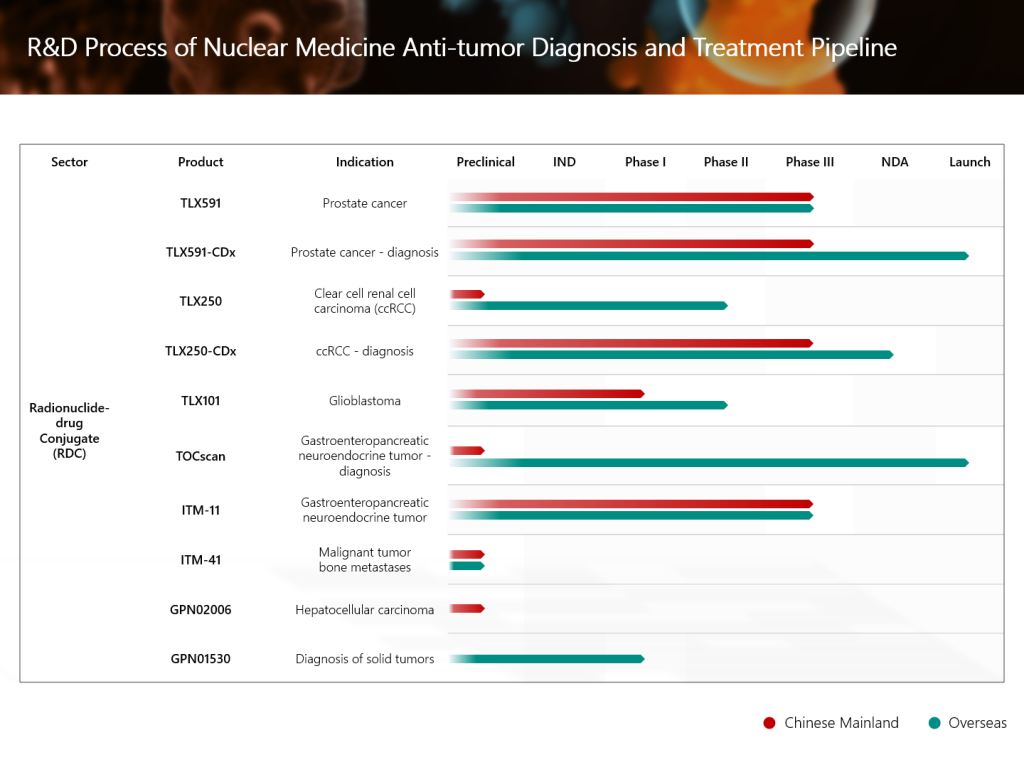
GPN01530, a globally innovative radionuclide-drug conjugate (RDC) independently developed by the Group, has recently been approved by the United States Food and Drug Administration (FDA) to conduct a Phase I/II clinical study for the diagnosis of solid tumors. It marks another solid step forward in the Group’s global strategy for nuclear medicine anti-tumor diagnosis and treatment.
- GPN01530 is a small molecule RDC drug that targets fibroblast activating protein (FAP). GPN01530 that independently developed by the Group optimizes the structure of the FAP ligand, improving its uptake in tumor tissues, while reducing its uptake in normal tissues.
- Compared to other FAP-targeted ligands, exhibits rapid tumor targeting, higher tumor uptake, and superior pharmacokinetic properties.
- The early research of the GPN01530 project was entirely independently developed by the Group based on its radiochemical labeling platform and animal molecular imaging platform at the Chengdu Radiopharmaceutical Base.
As the Group’s first self-developed RDC product that receives FDA approval for clinical trials, the successful approval of the GPN01530 clinical trial provides an important paradigm for the international development of the Group’s nuclear medicine product pipeline. It is a significant milestone in the Group’s global R&D and registration process for nuclear medicine anti-tumor diagnosis and treatment segment, fully demonstrating the Group’s comprehensive strength in the construction of cutting-edge nuclear medicine technology platforms, international clinical development, and registration applications, etc.
The Group will continue to advance the global R&D and registration of GPN01530 by relying on the international registration route of “dual filings in China and the United States”. Based on this, the Group will further deepen the globalization development strategy of this segment, actively promote the international clinical research and registration application of more independently developed innovative nuclear medicine products, and continuously enhance the Group’s core competitiveness and international influence in the field of nuclear medicine anti-tumor diagnosis and treatment.