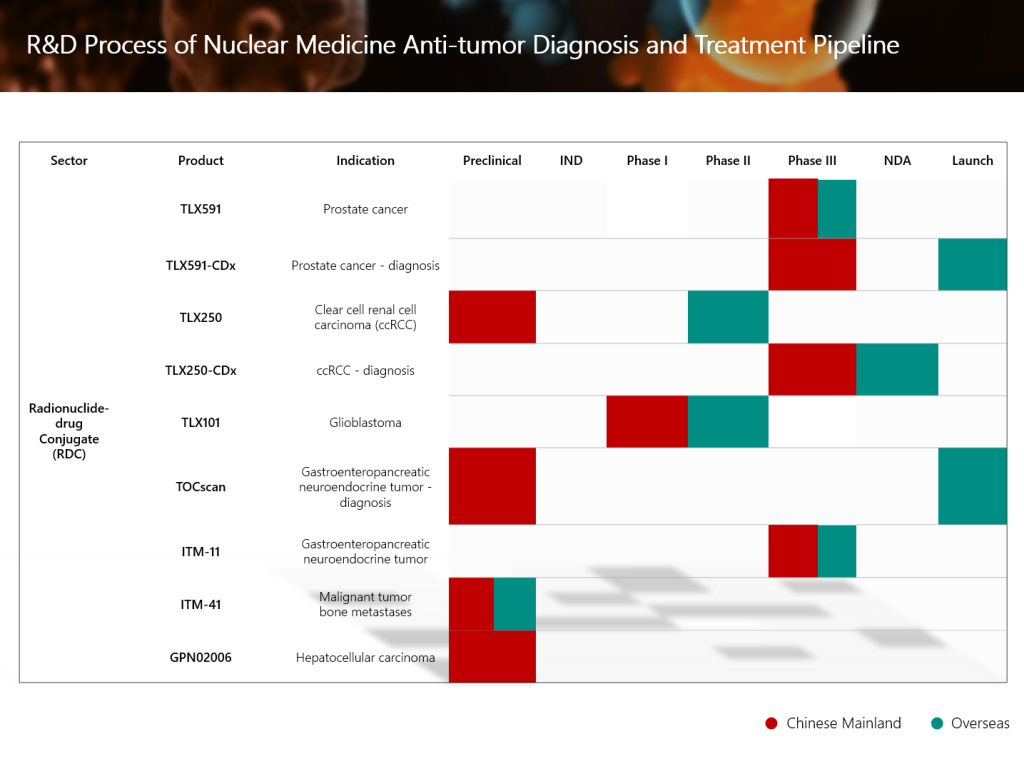
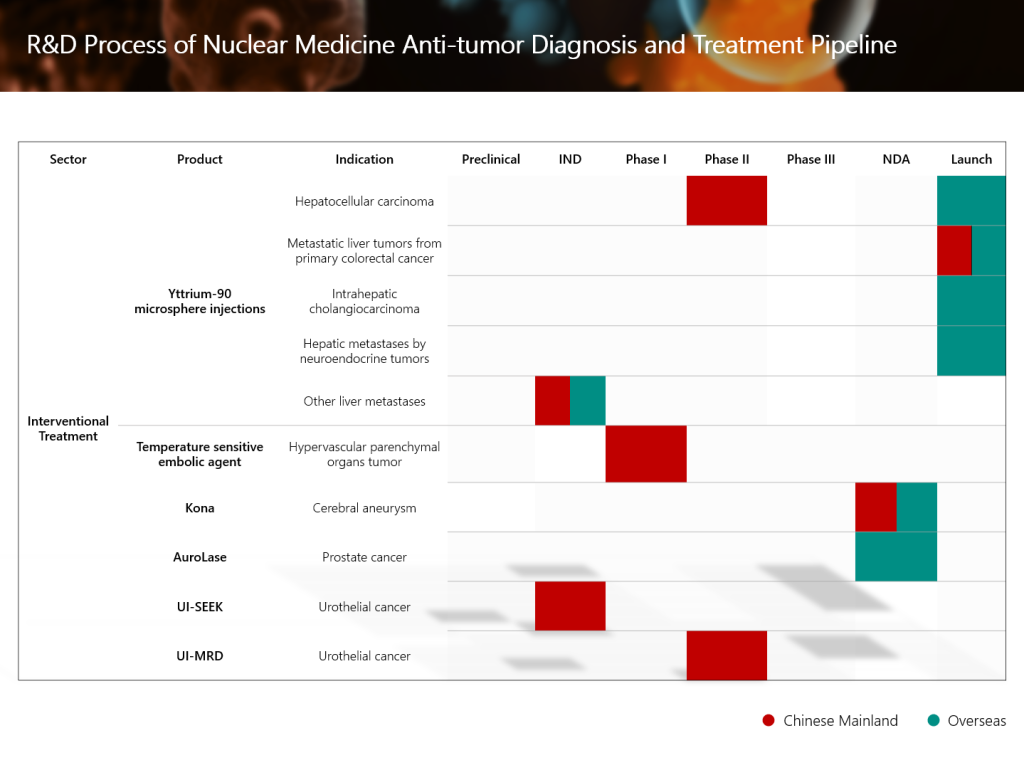
SIR-Spheres® Y-90 resin microsphere injection (YiGanTai®) of Sirtex Medical Pty Ltd, an associate company of Grand Pharma, has recently received CE Mark approval in Europe for new indications for the treatment of liver cancer patients. The approval of the new indications expands the scope of application of this therapy from unresectable hepatocellular carcinoma (HCC) and unresectable metastatic liver tumors from primary colorectal cancer (mCRC) to multiple indications, including unresectable intrahepatic cholangiocarcinoma (ICC), hepatic metastases by neuroendocrine tumors (mNET), or other liver metastases, covering a more comprehensive classification of primary liver cancer and secondary liver metastasis.
- The FDA’s approval of this new indication marks SIR-Spheres® Y-90 resin microsphere injection as the first and only FDA-approved selective internal radiation therapy for the dual indications of unresectable HCC and colorectal liver metastases.
- Relevant overseas clinical data will also provide key support for the expansion of liver cancer-related indications of the product in China. The Group is also actively collaborating with Chinese and international experts to develop other indications for SIR-Spheres® Y-90 resin microsphere injection. The Group will adopt an international registration pathway involving dual filings in China and the United States to facilitate global market expansion for the product.