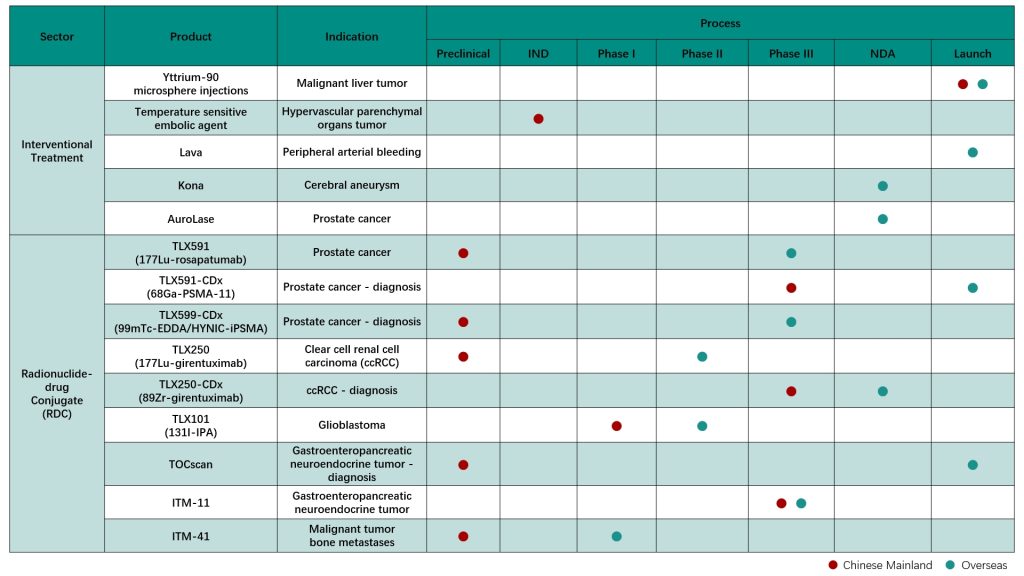
ITM-11, a global innovative radionuclide-drug conjugate (RDC) of Grand Pharmaceutical Group Limited (Grand Pharma, 0512.HK) for the treatment of gastroenteropancreatic neuroendocrine tumors, has been approved by the National Medical Products Administration of the People’s Republic of China (NMPA) recently to conduct Phase III clinical study (COMPOSE Study, NCT04919226) in China.
- The COMPOSE Study is a prospective, randomized, controlled, open-label, and international multi-center phase III clinical study. It will significantly promote the registration and R&D of ITM-11 in China, and further enhance the internationalization of the Group’s clinical development in the field of nuclear medicine anti-tumor diagnosis and treatment.
- The no-carrier-added 177Lu used by ITM-11 has higher specific activity and purity. The product has been granted orphan drug designation by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA). ITM-11 together with TOCscan®, another RDC product of the Group for the diagnosis of GEP-NETs, can form a product group to realize the integration of the diagnosis and treatment of GEP-NETs
- As for now, the Group has the largest total reserve of innovative diagnostic and therapeutic RDC drugs that have entered Phase III clinical studies in China, and also one of the innovative pharmaceutical companies in the world with the richest product pipeline and integrated diagnosis and treatment strategic plan in the field of nuclear medicine anti-tumor.