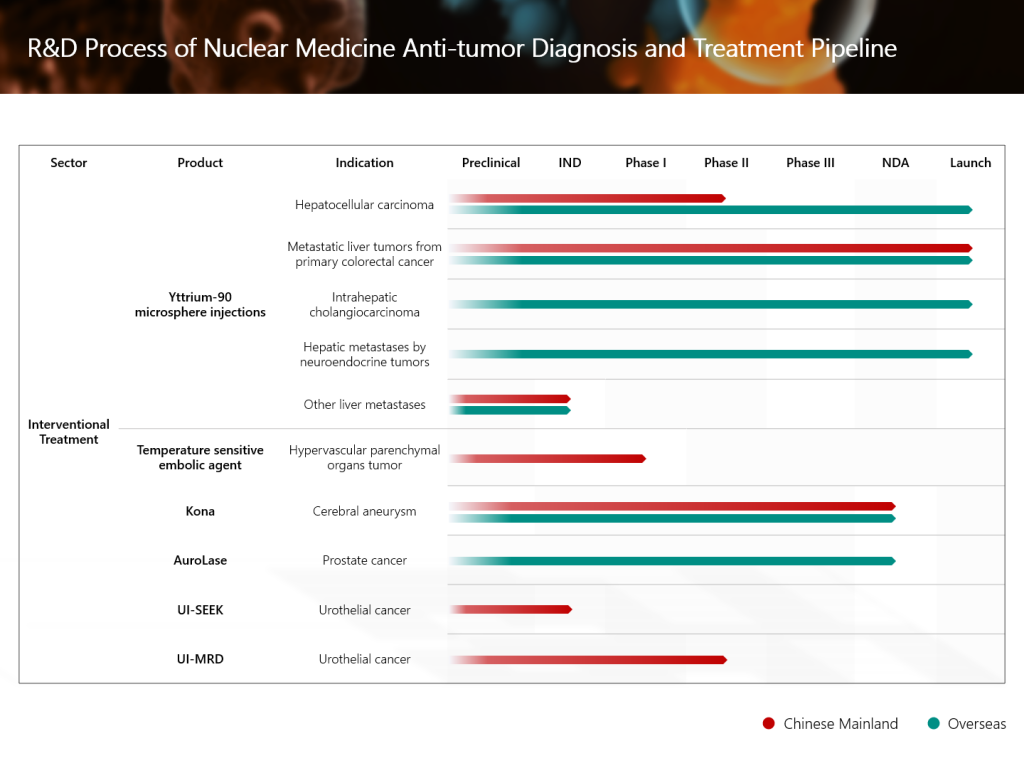
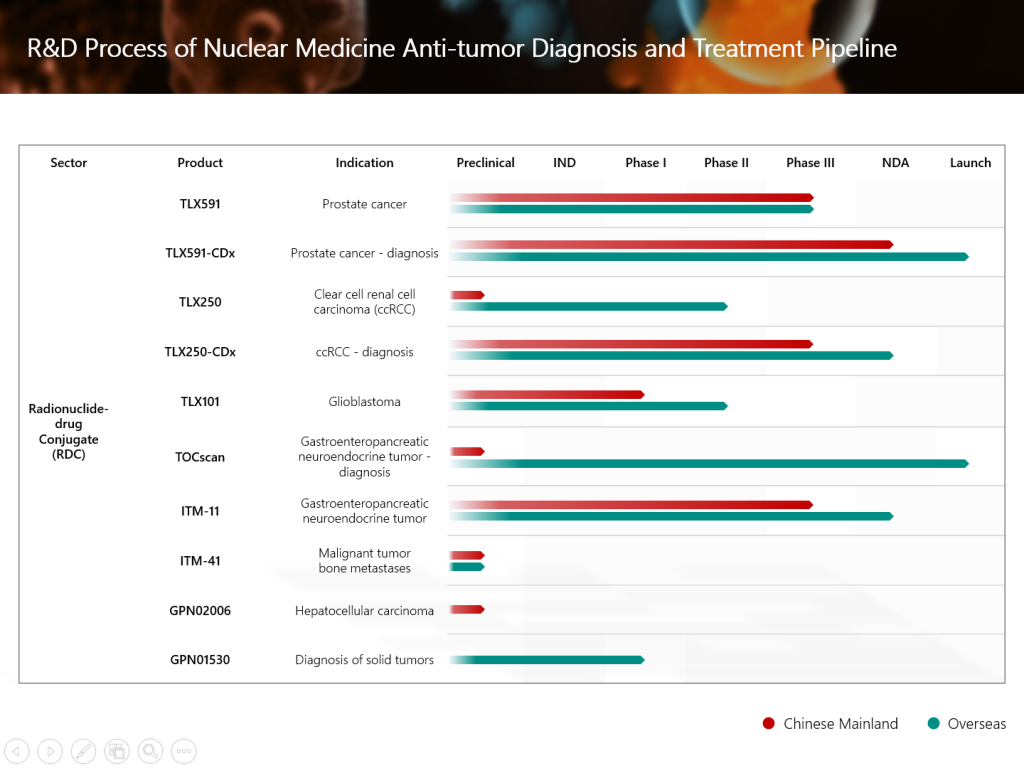
The new drug application (NDA) of TLX591-CDx (Illuccix®, gallium Ga 68 PSMA-11), Grand Pharma’s innovative investigational radionuclide-drug conjugate (RDC) for the diagnosis of prostate cancer, has recently been submitted to the National Medical Products Administration of the People’s Republic of China (NMPA) and has been accepted. This is a significant R&D advancement of Grand Pharma in the field of nuclear medicine anti-tumor diagnosis and treatment. In addition, Grand Pharma’s RDC product candidate TLX591 for the treatment of prostate cancer has been approved to conduct an international multi-center Phase III clinical study in China. In the future, the combination of the two therapeutic and diagnostic products builds up a head of steam, and are expected to bring more accurate and efficient diagnosis and treatment solutions to patients with prostate cancer in China.
- The NDA submission includes data from the TLX591-CDx clinical study in China, which reported positive top-line results in December 2025. The overall positive predictive value (PPV) was 94.8% for detection of tumors with TLX591-CDx (confidence intervals, CI: 85.9%-98.2%).
- TLX591-CDx has five major characteristics, including internalization into cells, stable biological activity, short circulation half-life in vivo, good permeability to tumor parenchyma, and rapid clearance by non-targeted tissues.
- In 2025, TLX591-CDx has been approved for commercialization in Austria, Belgium, Brazil, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Malta, Netherlands, Norway, Portugal, Slovakia, Spain, Sweden and the United Kingdom. The product achieved sales of approximately USD517 million in 2024; sales of approximately USD461 million in the first three quarters of 2025, representing an increase of more than 25% over the same period last year.