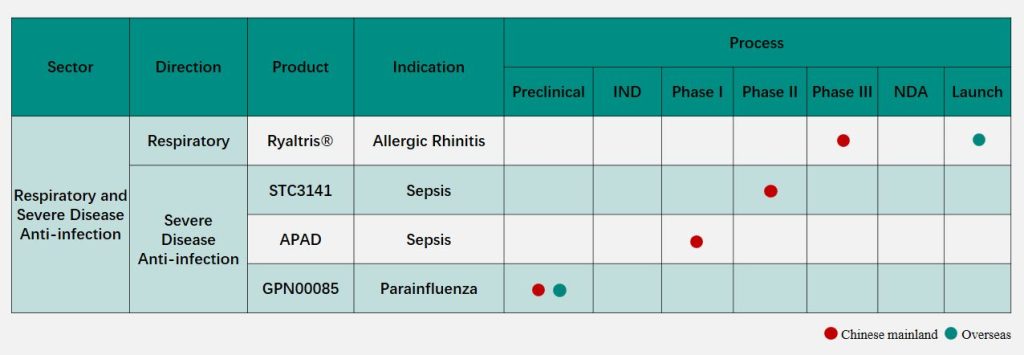
The Phase II clinical study for the treatment of sepsis in China of the global innovative drug STC3141, which is developed by the Grand Pharma Group Limited (0512.HK)’s wholly-owned subsidiary Grand Medical Pty Ltd. (an innovative drug R&D center set up by the Group in Australia), has recently completed first patient’s enrollment and dosing recently. This is another significant R&D progress for the Group in the field of respiratory and severe disease anti-infection.
The clinical study is a multi-center, randomized, double-blinded, placebo-controlled Phase II dose-exploring clinical study, aiming to evaluate the efficacy, safety and pharmacokinetic characteristics of STC3141 at different doses in patients with sepsis. The success of STC3141 in multiple clinical studies in the treatment of sepsis, ARDS and severe COVID-19 infection has revealed the favorable safety, tolerability and clinical benefit potential of this product in the treatment of severe diseases. STC3141 and APAD, another global innovative product for sepsis of the Group, complement each other in mechanism of action and are expected to form a good product portfolio in the treatment of severe diseases such as sepsis.