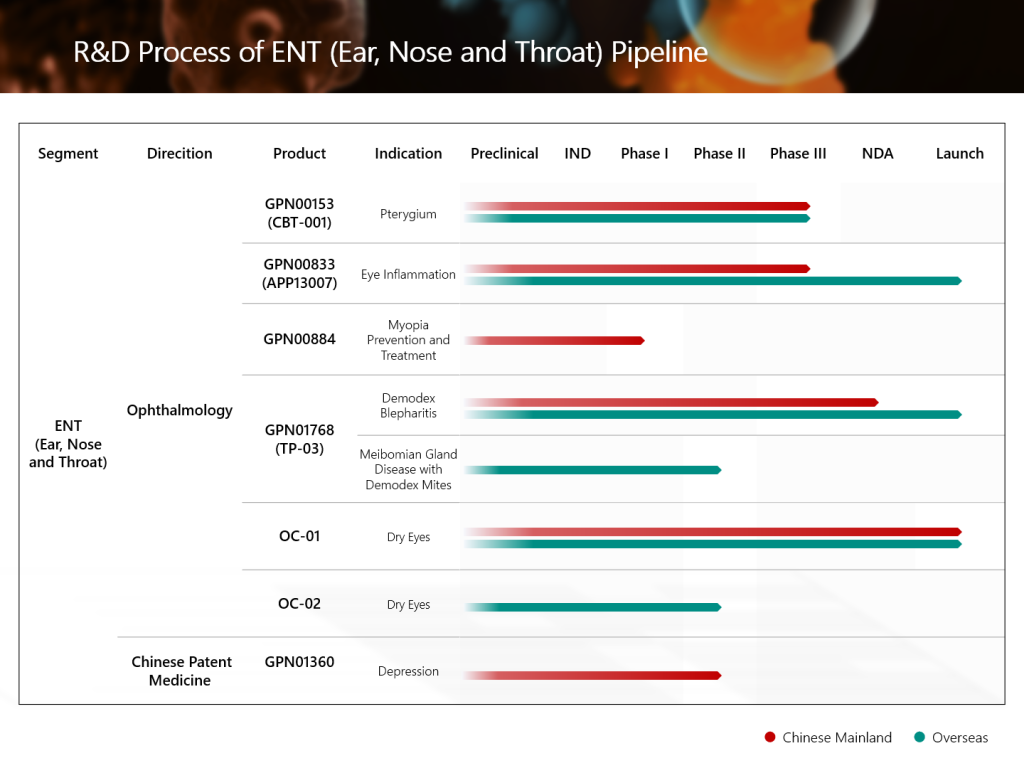
GPN01360 is a Class 1.1 innovative TCM for the treatment of depression (liver stagnation and spleen deficiency syndrome). Based on the ancient classic formula “Xiaoyaosan(逍遙散)”, it was developed and optimized through modern pharmacological and clinical research. The prescription consists of 12 TCM herbs, including Bupleurum, Turmeric Root Tuber, and Finger Citron, with the effects of soothing the liver and strengthening the spleen, relieving depression, and calming the mind. This TCM is mainly used to treat depression of the liver stagnation and spleen deficiency type, of which the typical symptoms include low mood, slow thinking, decreased willpower, irritability, anxiety, insomnia, forgetfulness, poor appetite, and fatigue.
- The results showed that after 8 weeks of treatment, the primary efficacy endpoint, HAMD-17, showed a significant change from baseline compared to placebo (P=0.0006<0.05).
- Secondary efficacy endpoints such as MADRS, TCM syndrome score, HAMD-17 remission rate and efficacy, and Hamilton Anxiety Scale (HAMA) all showed significant differences compared to placebo after 8 weeks of treatment (P<0.05).
- Preliminary human clinical studies involving nearly 100 people have shown that GPN01360 demonstrates favorable efficacy and safety in improving depressive symptoms, relieving anxiety and insomnia, and regulating spleen and stomach function.
- The success of the clinical trial marks a significant milestone for the Group in the direction of Chinese patent medicine in the field of the ENT (Ear, Nose, and Throat) segment.