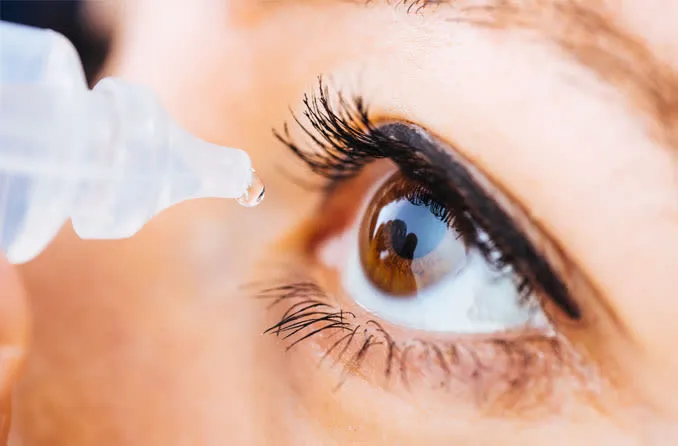
The Phase III clinical trial of GPN00833, an anti-inflammatory and analgesic hormone nanosuspension eye drop of the Group, conducted in China has completed and has successfully met clinical endpoint recently. It is another significant milestone for the Group in the direction of ophthalmology in field of ENT.
GPN00833 is anti-inflammatory and analgesic hormone nano-suspension eye drops. Its main active ingredient, clobetasol propionate, is a potent glucocorticoid, which has efficient local anti-inflammatory and strong capillary contraction effect.
It was approved to conduct a multi-center, randomized, double-blind, placebo-controlled, parallel group Phase III clinical study in China in April 2023, which enrolled 255 patients who underwent cataract surgery that randomly assigned to study drug group and placebo group in a 2:1 ratio, to evaluate the effectiveness and safety of GPN00833 in treating inflammation and pain after cataract surgery in the Chinese population.
In terms of overseas registration, the product was approved for commercialization by the U.S. Food and Drug Administration (FDA) in March 2024.
The Group always puts focus on the R&D of innovative products and advanced technologies. Adhering to a patient-centered and innovation-driven approach, the Group will continue to increase its investment in world-class innovative products and advanced technologies to meet unmet clinical needs and enrich its product pipeline and improve supply chain. The Group adopts the strategy of “global expansion and dual-cycle operation”, forming a new pattern of domestic and international cycles that synergize with each other. In this way, the Group can make full use of its industrial advantages and R&D capabilities, to accelerate the commercialization process for innovative products and provide patients with more advanced and diverse treatment options globally.





