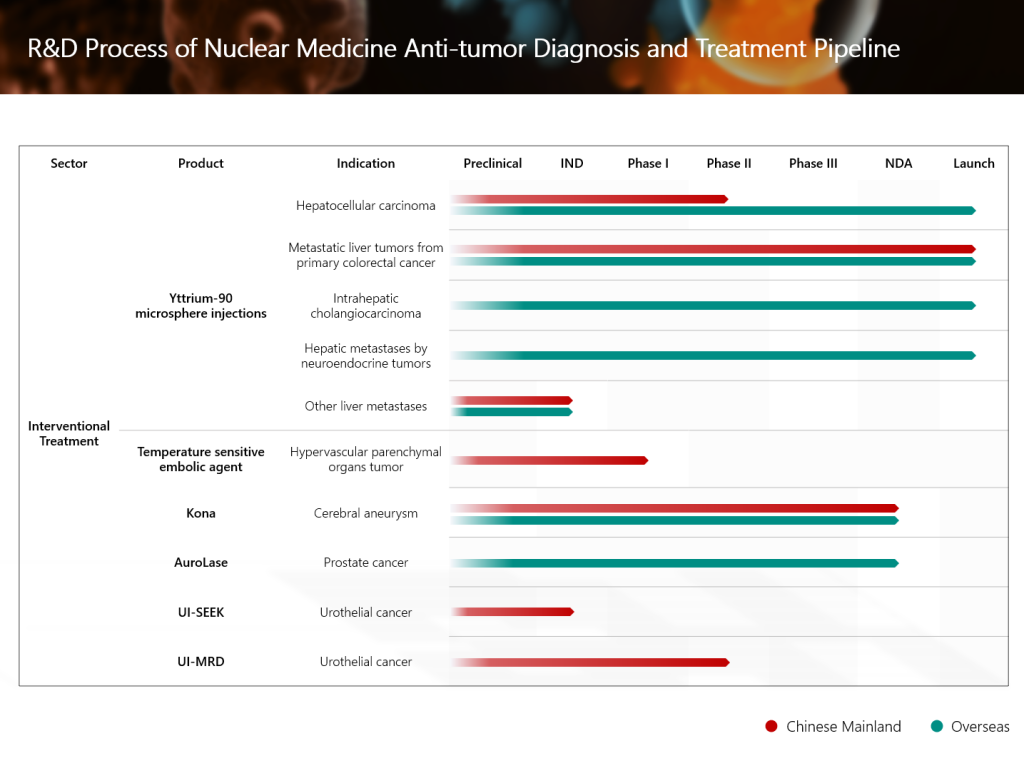
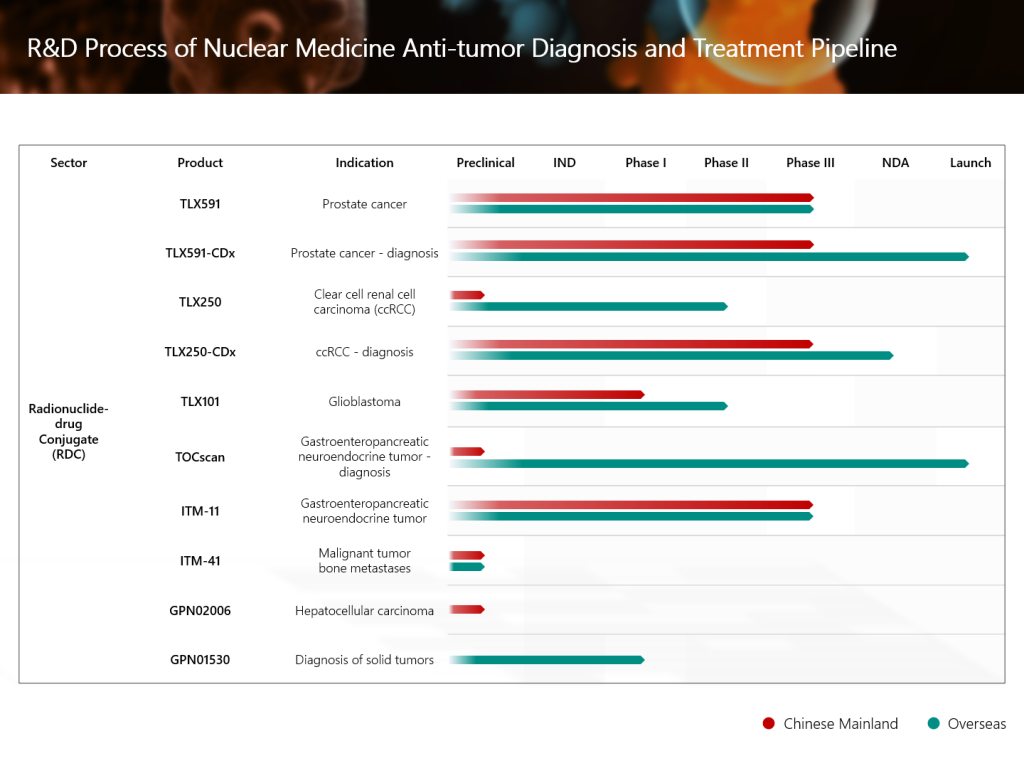
Grand Pharma’s innovative investigational radionuclidedrug conjugate (RDC) TLX591-CDx (Illuccix®, gallium Ga 68 PSMA-11) for the diagnosis of prostate cancer, has recently achieved positive top-line results from the Phase III clinical trial in China, and has successfully met the primary clinical endpoint. In addition, Grand Pharma’s RDC product candidate TLX591 for the treatment of prostate cancer has been approved to conduct an international multi-center Phase III clinical study in China. In the future, the combination of the two therapeutic and diagnostic products builds up a head of steam, and are expected to bring more accurate and efficient diagnosis and treatment solutions to patients with prostate cancer in China.
- Based on available published data, TLX591-CDx has five major characteristics, including internalization into cells, stable biological activity, short circulation half-life in vivo, good permeability to tumor parenchyma, and rapid clearance by non-targeted tissues.
- According to top-line clinical results, the overall positive predictive value (PPV) was 94.8% for detection of tumors with TLX591-CDx (confidence intervals, CI: 85.9%-98.2%).
- Patients with suspected biochemical recurrence (BCR) were assigned to groups according to their baseline prostate specific antigen (PSA) level in this trial. TLX591-CDx PET imaging demonstrated high PPV in all patient groups.