SIR-Spheres® Y-90 resin microsphere injection (YiGanTai®) of Sirtex Medical Pty Ltd, an associate company of Grand Pharma, has recently received official approval from the United States Food and Drug Administration (FDA) for a new indication for the treatment of unresectable hepatocellular carcinoma (HCC).

The interim data show that, DOORwaY90 successfully met its prespecified co-primary endpoints: the objective response rate was as high as 98.5% as assessed by independent central review; all evaluable patients demonstrated treatment response, indicating a 100% local tumor control rate; additionally, the median duration of response exceeded 300 days.
Based on the above breakthrough interim clinical data, the FDA formally approved SIR-Spheres® Y-90 resin microsphere injection for the indication of unresectable HCC ahead of schedule, without any restrictions on tumor diameter size.
This early approval was based on the breakthrough interim data from the DOORwaY90 clinical trial that successfully met the pre-specified co-primary endpoints, marking SIR-Spheres® Y-90 resin microsphere injection the world’s first and only selective internal radiation therapy product approved by the FDA for dual indications of both unresectable HCC and colorectal cancer liver metastases (CRLM).
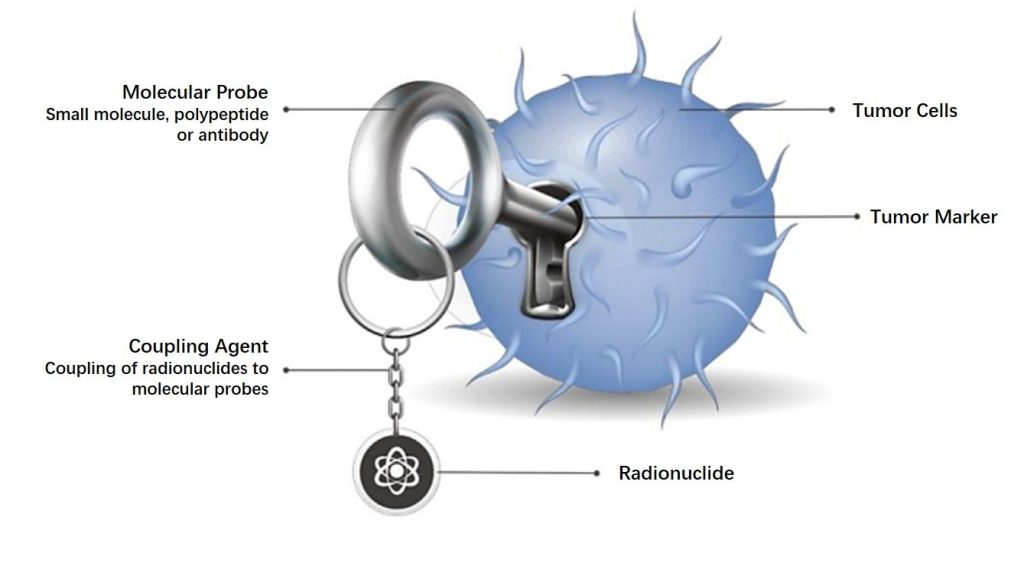
The nuclear medicine anti-tumor diagnosis and treatment platform is the Group’s high-end
technology platform in the field of anti-tumor. The Group has achieved a comprehensive
strategic plan in the fields of R&D, production, sales, regulatory qualifications and established
a complete industrial chain.
The Group always puts focus on the R&D of innovative products and advanced technologies.
Adhering to a patient-centered and innovation-driven approach, the Group will continue to
increase its investment in world-class innovative products and advanced technologies to meet
unmet clinical needs and enrich its product pipeline and improve supply chain. The Group
adopts the strategy of “global expansion and dual-cycle operation”, forming a new pattern of
domestic and international cycles that synergize with each other. In this way, the Group can
make full use of its industrial advantages and R&D capabilities, to accelerate the
commercialization process for innovative products and provide patients with more advanced
and diverse treatment options globally.