On December 22-24, 2023, the 8th Annual of Chinese Interventional Neuroradiolcy Society of CSA (CINS) was held in Beijing. As an important platform for academic exchanges on neurological interventions in China, this conference based on the forefront of neurological interventions, adheres to the concept of “intervention, innovation, collaboration for win-win”.
The conference gathered famous neurointervention experts all over the world, focusing on current research trends and in-depth clinical technical difficulties. Grand Pharma has brought its core neurointerventional products to this conference, bringing innovative vitality to China’s neurointervention development.

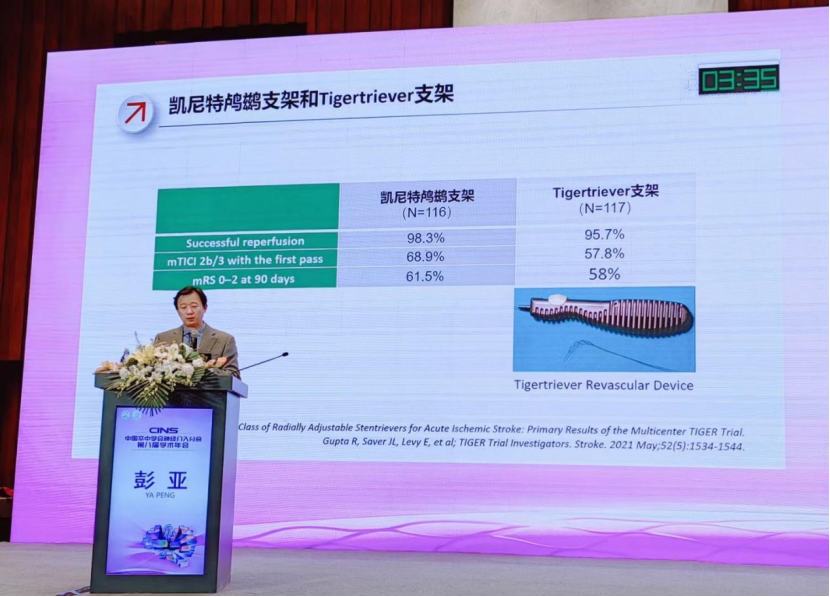
Grand Pharma presented the clinical study data of KEYNEUT LUCI® adjustable stent retriever, a leading neurointerventional product of the company, at the symposium of CINS 2023. Prof. Peng Ya (PI), the principal research of the clinical trial, gave a profound presentation of the clinical registration study data of KEYNEUT LUCI® (鸬鹚) and shared the clinical application experience. It provides strong evidence-based support for the clinical safety and efficacy of KEYNEUT LUCI® in the domestic market in the future.