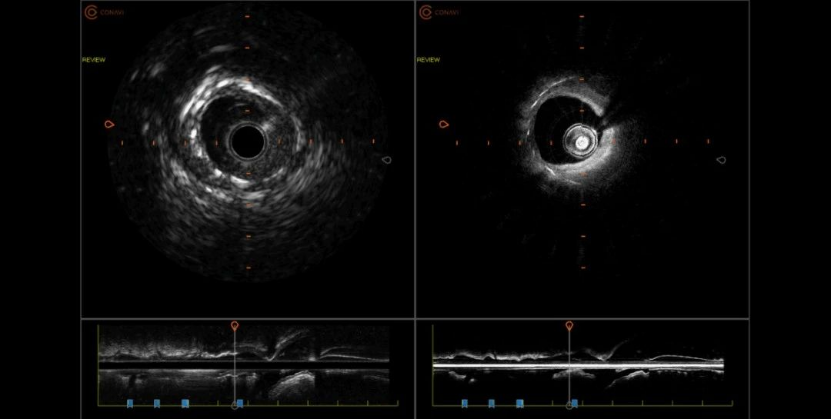
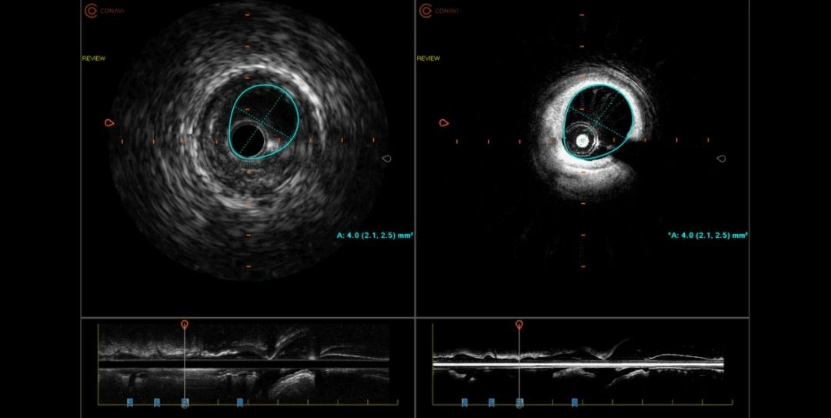
On November 29, 2023, Professor Zheng Xiaoqun’s team from the Department of Cardiology at Dalian Municipal Central Hospital completed the first clinical application of the NOVASIGHT™ HYBRID SYSTEM coronary imaging system in Northeast China after its launch.

Through the combined application of intravascular ultrasound (IVUS) + optical correlation tomography scanning (OCT) 2-in-1 intravascular imaging technology, it efficiently provides accurate and comprehensive intravascular imaging information. This is also the first clinical application of Grand Pharma’s coronary imaging device NOVASIGHT.

NOVASIGHT™ HYBRID SYSTEM, an innovative IVUS+OCT dual-mode imaging system (device + catheter), was approved for marketing by the National Medical Products Administration (NMPA) this year. It is also the only IVUS+OCT dual-mode imaging system that has been approved by the U.S. FDA, and is already in clinical use in the U.S. and Canada.