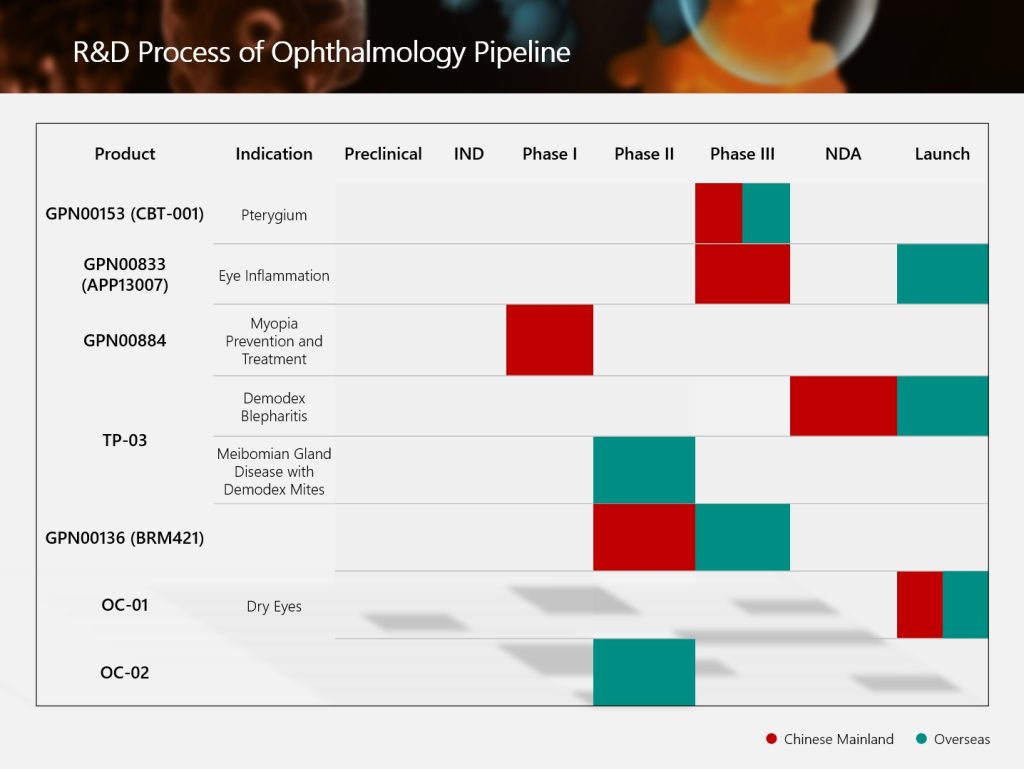
The Group’s global innovative ophthalmic product GPN01768 (TP-03, lotilaner ophthalmic solution, 0.25%) for the treatment of Demodex blepharitis has been approved for commercialization by the Pharmaceutical Administration Bureau of Macao Special Administrative Region of China (MACAO ISAF) recently.
GPN01768 was developed by Tarsus Pharmaceuticals, Inc. (Tarsus) and is the first and only product approved by the United States Food and Drug Administration (FDA) for the treatment of Demodex blepharitis.
There are positive topline results of GPN01768 Phase II clinical research in the United States for the treatment of MGD in patients with Demodex mites.
The product’s approval by MACAO ISAF has laid the foundation for its future approval in the Guangdong-Hong KongMacao Greater Bay Area, and is also expected to further promote its implementation in Mainland China.