The Group’s world-leading nuclear medicine R&D and production base located in Wenjiang District, Chengdu, has obtained a Class A “Radiation Safety License” issued by the Ministry of Ecology and Environment of the People’s Republic of China recently, and will officially start operations in June this year. This base was signed at the end of 2022, the environmental impact assessment was fully conducted in April 2023, the construction permit was obtained and the official groundbreaking ceremony was held in November of the same year. The main structure was completed in just five months, breaking the industry construction record with a two-year cycle. The base will help the Group improve its global footprint in nuclear medicine and build a new independent and controllable ecosystem.
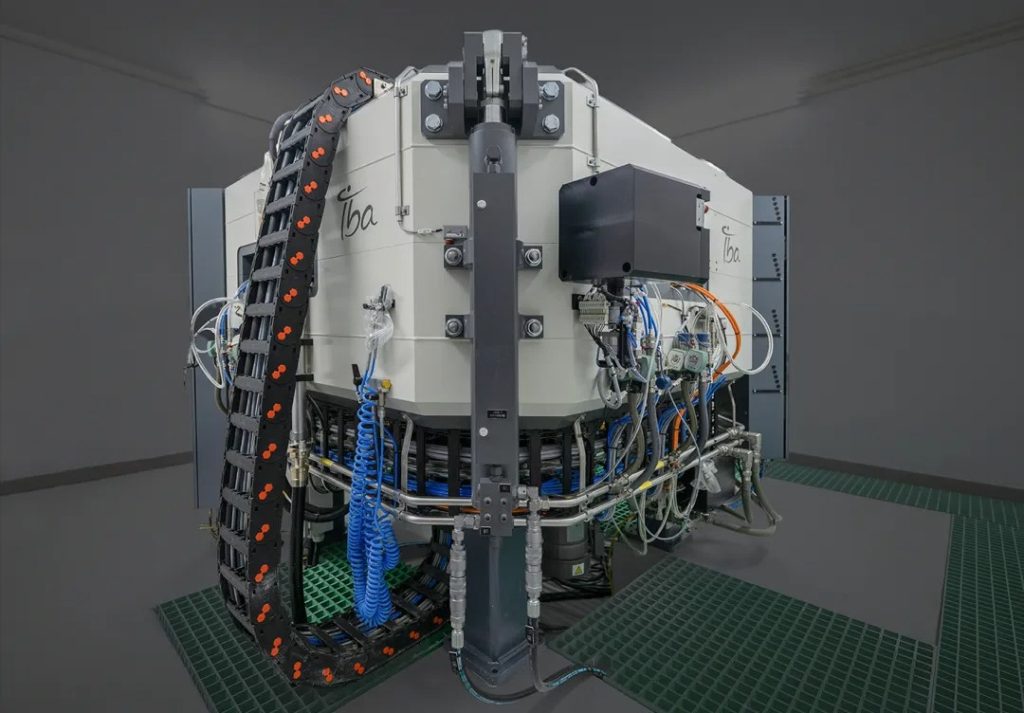
- As the world’s first closed-loop platform for the entire nuclear medicine industry chain, focusing on core areas such as isotope process development and preparation, nuclear medicine coupling technology, and automated labeling technology. It covers the one-stop full life cycle management of nuclear medicine early research, process development, quality research, non-clinical research, intelligent production and precise distribution, establishing a world-class R&D, production, quality and operation system.
- The base has 14 production lines that meet the requirements of Good Manufacturing Practice (GMP) for pharmaceutical production, and has built a full-chain AI operation system and intelligent manufacturing system, which can realize the independent production of multiple isotopes and multiple nuclear medicine preparations. At the same time, a production line for α-nuclide drugs is reserved, which is one of the smart factories with the most complete types of nuclides and the highest degree of automation in the world. It can fully meet the Group’s needs for multi-variety and large-scale preparation of therapeutic and diagnostic nuclear medicines.
- The nuclear medicine R&D and production base has ten highlights and technological breakthroughs:
- Realize full-link AI intelligent operation, greatly improve operational efficiency;
- Equipped with the highest international standard radioactive operation hot room to completely block the leakage path of radioactive materials;
- Create the world’s first full-process radiation monitoring system in the field of radioactive drugs to achieve “zero external discharge” and “zero exceeding standards”;
- The “four-in-one” of “ligand + nuclide + technology platform + intelligent production” breaks the international monopoly. These highlights and technological breakthroughs not only meet the standards of the International Atomic Energy Agency (IAEA), but also set a new safety benchmark for the nuclear medicine industry.