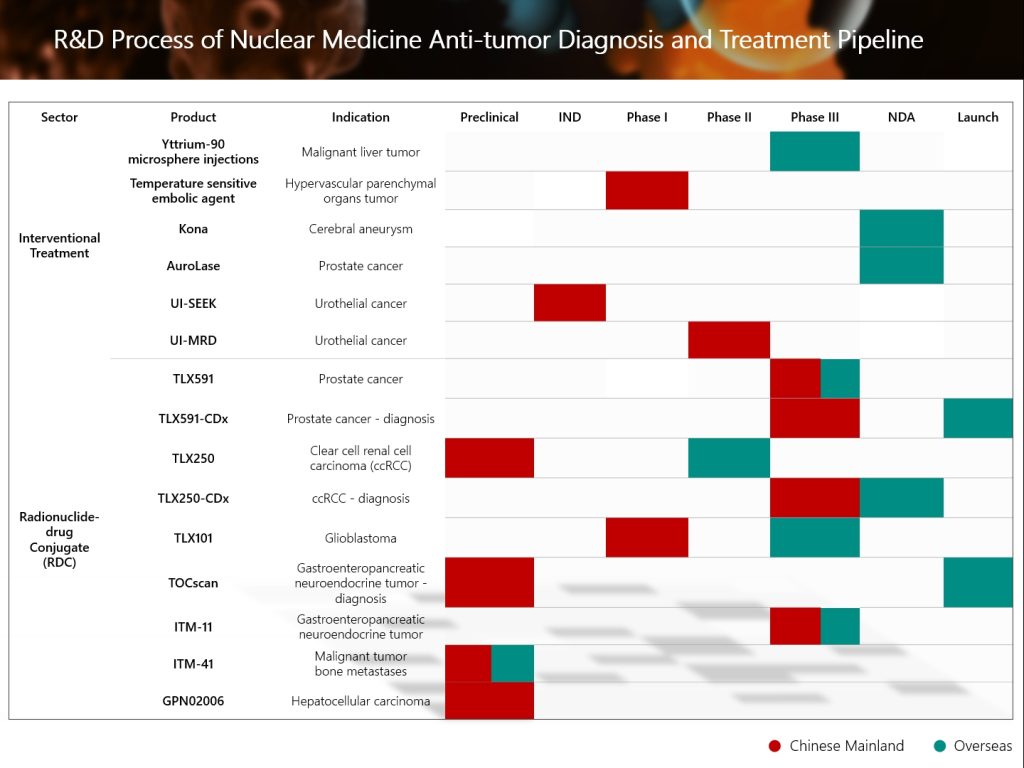
The Group’s global innovative radionuclide-drug conjugate (“RDC”) TLX591 for the treatment of prostate cancer, has recently had its Investigational New Drug (IND) application for inclusion in an international multicenter Phase III clinical trial formally accepted by the the National Medical Products Administration of the People’s Republic of China (“NMPA”). This represents a significant R&D progress for the Group in the field of nuclear medicine anti-tumor diagnosis and treatment.
- As the prostate cancer treatment field urgently needs solutions with lower radiation exposure, TLX591, with its precise targeting and differentiated pharmacological advantages, has demonstrated clinical potential to surpass existing PSMA-targeted small-peptide RLT molecules. It is expected to redefine the treatment standard for PSMA-positive mCRPC.
- The Phase III clinical trials of TLX591 and TLX591-CDx in China are critical to the Group to achieve comprehensive coverage of “integrated diagnosis and treatment” of prostate cancer, which is expected to deliver more precise and effective diagnostic and therapeutic solutions for patients with prostate diseases in China.
- The Group attaches great importance to the global development strategy of the nuclear medicine industry, actively promotes the global development and registration process of innovative nuclear medicine products, and will continuously deepen the global expansion of the Group’s nuclear medicine product pipeline.