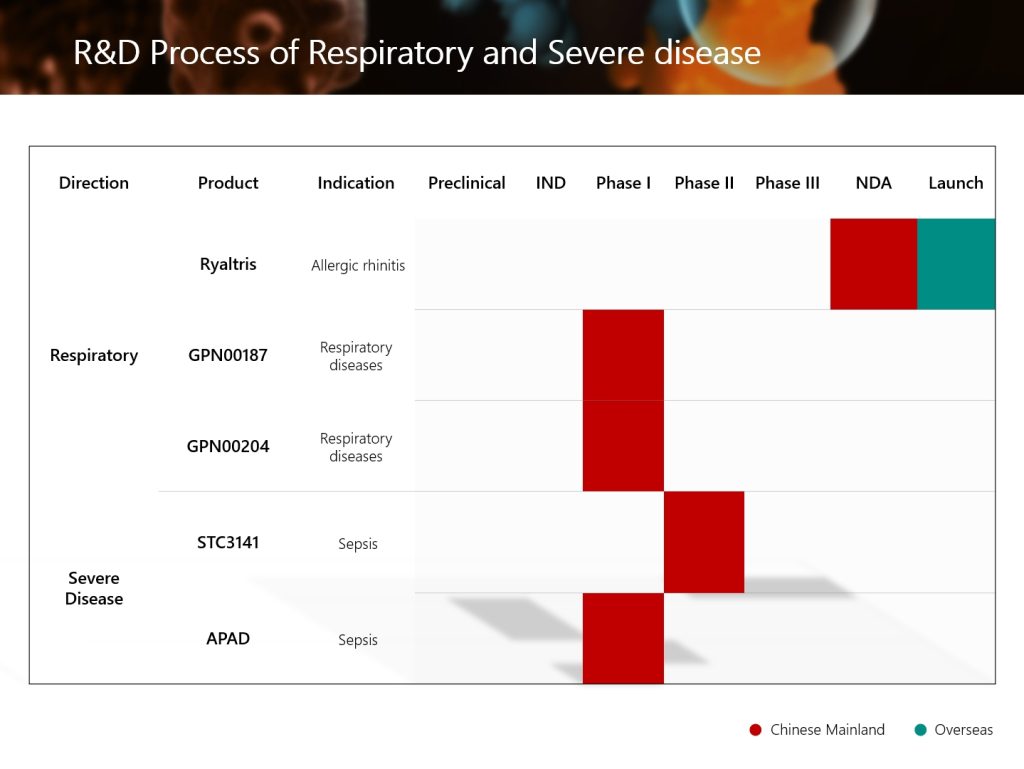
The Phase II clinical study for the treatment of sepsis in China of the global innovative drug STC3141, which is developed by the Group’s wholly-owned subsidiary Grand Medical Pty Ltd. (an innovative drug R&D center set up by the Group in Australia), has successfully reached the clinical endpoint. The progress of this clinical study is at the forefront of global sepsis research. This is another significant R&D progress of the Group in the field of severe and critical diseases.
At the same time, STC3141 is the first sepsis treatment program centered on rebuilding immune homeostasis in the world, achieving a major upgrade in the treatment dimension. On the basis of existing symptomatic supportive treatments such as anti-infection, fluid resuscitation, and maintaining organ function, it precisely regulates the core cause of the disease, immune disorder, to help the body restore balance, filling the current clinical gap in etiology-oriented treatment of sepsis. The success of this clinical study is expected to usher in a new era of sepsis treatment.
- The results showed that the SOFA scores of the drug treatment groups on the 7th day were significantly lower than those of the baseline, especially in the high-dose group, where the decrease was significantly greater than that in the placebo group. The difference was statistically significant and clinically significant.
- The trend of the secondary endpoints was consistent with the primary endpoints, which was in line with expectations. In addition, STC3141 has favorable safety and tolerability, and its pharmacokinetic characteristics are also in line with expectations. The results confirmed the effectiveness and safety of STC3141 in the treatment of sepsis, bringing new breakthroughs in the field of critical care.
- STC3141 is the first sepsis treatment program centered on rebuilding immune homeostasis in the world, achieving a major upgrade in the treatment dimension.