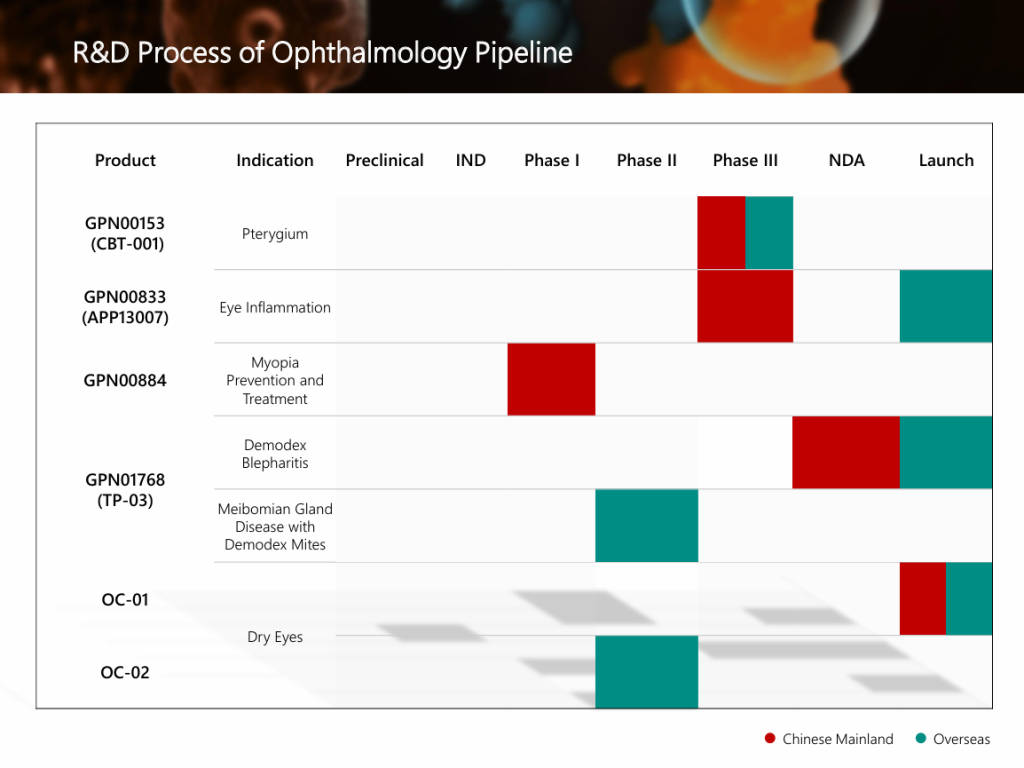
The Phase IIa clinical study conducted in China of Grand Pharma’s global innovative ophthalmic drug GPN00884 used to delay the progression of myopia in children, has completed the first patient enrollment recently. This study is a randomized, double-blind, placebo parallel-controlled Phase IIa clinical trial, planning to enroll more than 80 myopic subjects aged 6 to 12 years old. It aims to preliminarily evaluate effectiveness of GPN00884 eye drops in delaying the progression of myopia in children and its safety in children with myopia.
- GPN00884 eye drops are an innovative drug with a new mechanism used to delay the progression of myopia in children. Compared with low-concentration atropine eye drops, GPN00884 eye drops have no mydriasis effect, will not cause adverse reactions such as photophobia and decreased accommodation, and the dosing period is not limited, which can improve patient compliance.
- China is the country with the most myopia in the world. According to the survey results of the National Health Commission, the prevalence of myopia among adolescents in China ranked first in the world. In 2022, the overall myopia rate of children and adolescents in China was 51.9%.
- At present, there is still a lack of drugs with clear efficacy and safety in terms of delaying the progression of myopia in children in China, indicating an unmet clinical need in the field of this disease. GPN00884 eye drops are expected to provide doctors and patients with a new clinical treatment solution for delaying the progression of myopia in children.