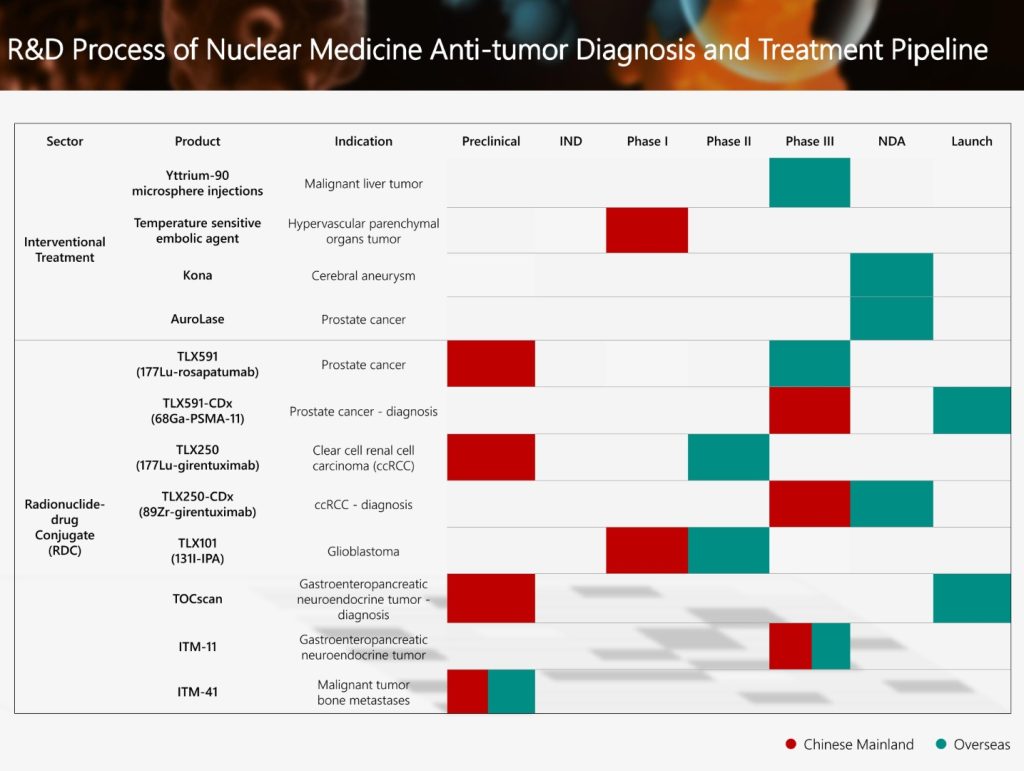
A Phase III clinical study application of ITM-11 (COMPETE Bridging Study), a global innovative radionuclide-drug conjugate (RDC) of the Group for the treatment of gastroenteropancreatic neuroendocrine tumors, has been accepted by the National Medical Products Administration of the People’s Republic of China (NMPA) recently. The COMPETE Bridging Study is a prospective, randomized, controlled, open-label, and multi-center Phase III clinical study, planning to enrol more than 60 patients in China. It aims to evaluate the efficacy and safety of ITM-11 Peptide Receptor Radionuclide Therapy (PRRT) in patients with inoperable, progressive, Grade 1 or Grade 2 well-differentiated, somatostatin receptor-positive (SSTR+) gastroenteropancreatic neuroendocrine tumors (GEP-NETs) compared with standard therapy. In March this year, the Group obtained approval from the NMPA to conduct a Phase III clinical trial of ITM-11 for aggressive Grade 2 or Grade 3 well-differentiated GEP-NETs (COMPOSE Trial), which is in progressing. The COMPETE and COMPOSE Trials are targeting the broad population of GEP-NET patients who are expected to benefit from treatment with ITM-11.
ITM-11 is a therapeutic RDC drug based on radionuclide conjugated technology that targets GEP-NETs. It conjugates no-carrier-added 177Lu with a somatostatin analog, and targets the killing of tumor cells by binding to the somatostatin receptor (SSTR) that is highly expressed on the surface of GEP-NET tumors. Compared with the commonly used carrier-added 177Lu radioisotope products, the no-carrier-added 177Lu has higher specific activity and purity, and produces less long half-life impurities during the production process and so has easier handling of radioactive waste. ITM-11 has been granted orphan drug designation by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) among others.