Grand Pharma’s first commercial prescription in Mainland China of Varenicline Tartrate Nasal Spray (“OC-01”), the world’s first nasal spray product to increase tear secretion in patients with dry eye disease, was issued in the First Affiliated Hospital, Sun Yat-sen University and Shenzhen Eye Hospital after its official approval. This approval milestone marks the availability of a new treatment option and the only nasal spray product currently approved to increase tear secretion in patients with dry eye disease in China and has officially entered clinical application, providing a new treatment option for Chinese dry eye patients.
- This product was approved in the United States in October 2021, and is currently the world’s first and only preservative-free, multi-dose, sterile packaged nasal spray approved to treat the signs and symptoms of dry eye disease in patients.
- OC-01 is a highly selective nicotinic acetylcholinergic receptor agonist. It is believed to increase tear secretion in patients with dry eye disease by activating the trigeminal parasympathetic pathway and increasing basal tear secretion.
- Artificial tears are the most commonly used treatment option, while OC-01 only needs to be administered as a nasal spray twice a day, which may avoid the cumbersome use of traditional eye drops, thereby it may improve patient compliance.
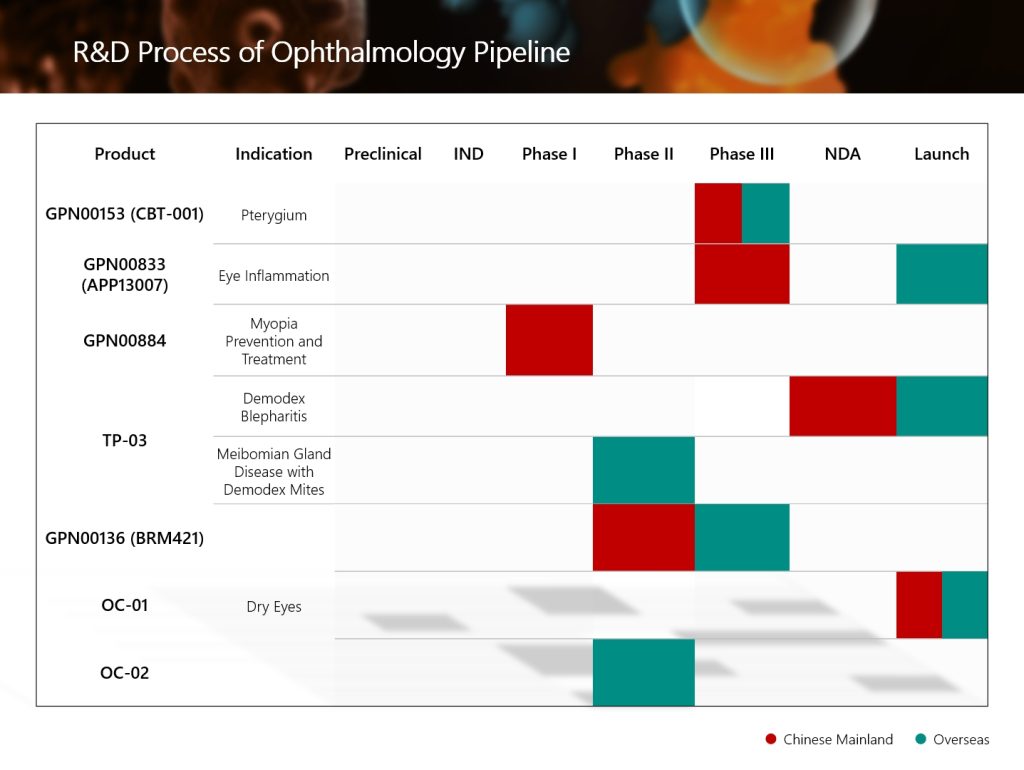
Among them, the global innovative ophthalmic product GPN01768 (TP-03, lotilaner ophthalmic solution, 0.25%) for the treatment of Demodex blepharitis has had its new drug application submitted to the NMPA and was accepted, and was approved for commercialization by the Pharmaceutical Administration Bureau of Macao Special Administrative Region of China in May 2025; GPN00833, a hormone nanosuspension eye drops for anti-inflammatory and analgesic, has completed the Phase III clinical trial in China and met the primary endpoint in November 2024; GPN00153 (CBT-001), an innovative modified new drug for the treatment of pterygium, has had its Phase III clinical study in China completed the first patient enrollment in March 2024, and has had its international multi-center Phase III clinical study completed all patients enrollment in June 2025; GPN00884, a global innovative ophthalmic drug used to delay the progression of myopia in children, has completed the first patient enrollment in the Phase I clinical study conducted in China in June 2024. In the future, the Group will accelerate the comprehensive and differentiated strategic plan of its R&D pipeline, and continuously enrich its innovative product reserves in the ophthalmic segment.
In addition, the Group has attracted and trained a group of professionals with both clinical and marketing experience in the field of ophthalmology, and established a professional marketing team that is customercentric and academic-led. It has also established long-term and stable cooperation with large pharmaceutical distribution companies and chain pharmacies, forming a nationwide marketing network. As innovative ophthalmic products continue to be approved, the Group will make full use of its core advantages in this field, continue to explore the cutting-edge innovation track of ophthalmology, further strengthen the professional promotion and brand building of core products, and provide new momentum for the Group’s sustainable and healthy development.
The Group always puts a high value on the R&D of innovative products and advanced
technologies. Sticking to patient-centered and innovation-driven, the Group will continue to
increase its investment in world-class innovative products and advanced technologies to meet
unmet clinical needs and enrich product pipeline and improve supply chain. The Group adopts
the strategy of “global expansion and dual-cycle operation”, forming a new pattern of domestic
and international cycles that synergize with each other. In this way, the Group can make full
use of its industrial advantages and R&D capabilities, to accelerate commercialization process
for innovative products and provide patients with more advanced and diverse treatment options
in the world.